Care to Cure
From ethical care to medical innovation — a complete biomedical value chain connecting Mauritius and the United States.
The Bioculture Group integrates every stage of preclinical and translational research. Our Care to Cure model unites ethical animal welfare, scientific excellence, and operational compliance into one continuous system — ensuring that the science we enable is humane, reliable, and impactful.
The Biomedical Value Chain
The “Care to Cure” Approach:
Bioculture’s integrated ecosystem bridges the gap between ethical animal care and advanced biomedical innovation.
This model creates a seamless pathway — from responsible primate breeding in Mauritius to preclinical gene therapy research in the United States — ensuring consistency, traceability, and scientific integrity throughout the entire research process.

Care
Humane breeding, enrichment, and welfare-driven management of SPF macaques.

Supply
Humane breeding, enrichment, and welfare-driven management of SPF macaques.

Care
Ethical and fully regulated export, quarantine, and logistics through Bioculture Mauritius and BCUS LLC.
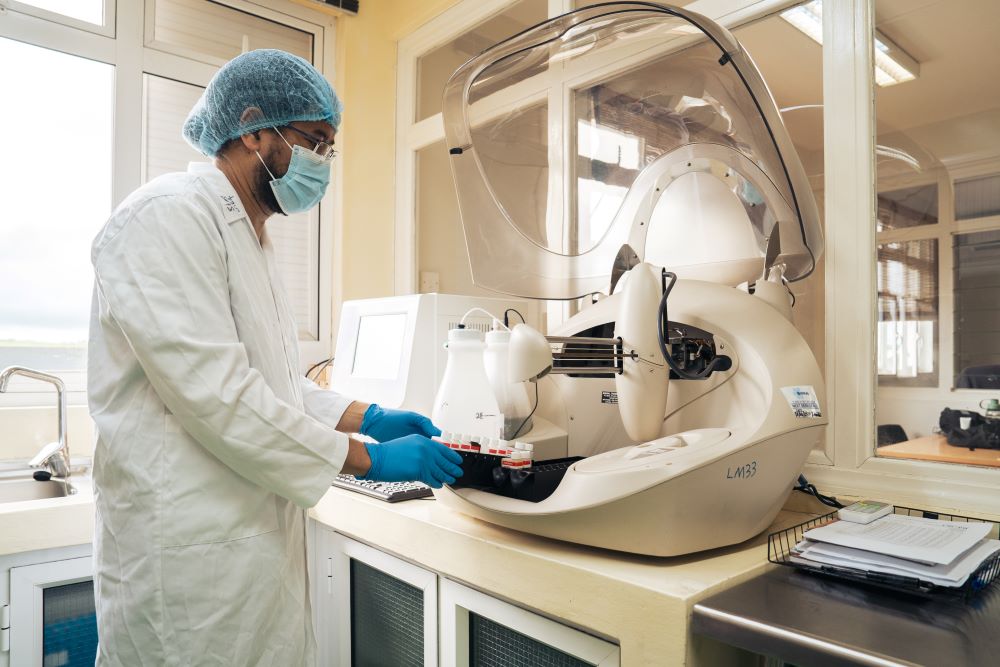
Research
Preclinical and genetic research through Franklin Biolabs and AnewCRO.
Our Services
Bioculture Group operates through two primary service categories that together define our biomedical value chain:
Supply of Non-Human Primates (NHPs):
Ethical breeding, housing, and export of SPF cynomolgus macaques for global research.

Research Services
Preclinical, translational, and genetic research programs supporting discovery and regulatory development.

Supply of NHPs
Bioculture is one of the world’s most respected sources for ethically bred cynomolgus macaques (Macaca fascicularis).
Our SPF (Specific Pathogen Free) colonies in Mauritius represent over 40 years of scientific, genetic, and welfare management.
Importance in Biomedical Research:
Non-human primates remain an essential model for translational medicine. Their physiological similarity to humans provides crucial insight into vaccine development, pharmacokinetics, and toxicology — enabling safer, more effective therapies.
Ethics and Quality:
Every Bioculture primate is raised under internationally certified conditions that prioritize behavioral enrichment, socialization, and positive reinforcement training.
All breeding, transport, and holding operations comply with AAALAC International, USDA, CDC, and Mauritian regulatory standards.

Research Services
Through Franklin Biolabs and AnewCRO, Bioculture provides advanced research capabilities spanning preclinical toxicology, pharmacology, and genetic medicine.
This expansion allows the Group to contribute directly to the discovery and validation of next-generation therapies.
Types of Research Supported:
-Cell and Gene Therapy: AAV, RNA, and viral vector studies supporting IND submissions.
-Biologics and Vaccines: Preclinical safety, PK/PD, and immunogenicity testing.
-CNS and Ocular Research: Specialized surgical and behavioral models in NHPs.
-Discovery Toxicology: Non-GLP studies for candidate selection and early screening.
Scientific Expertise:
Bioculture’s research entities are staffed by leading scientists and CRO professionals with decades of experience in translational research and regulatory compliance.
By combining ethical animal sourcing with controlled laboratory precision, we deliver reproducible data and shorten development timelines for global clients.
Subsidiary Links:
[Franklin Biolabs →] Gene and cell therapy CRO.
[AnewCRO →] NHP-based preclinical research.
Certifications & Compliance
Bioculture Group’s commitment to quality and integrity is embedded in every process — from breeding to data reporting. Our global compliance framework ensures that all operations meet or exceed international research and welfare standards.

Animal care and use
accreditation.

U.S. import and acclimation compliance.

U.S. research and welfare oversight.

Licensing and biodiversity conservation.
Industries We Serve
Partnering in drug discovery, biologics, and vaccine development.

Providing preclinical expertise for gene and cell therapy innovation.

Supporting translational research and ethical scientific training.

Collaborating on infectious disease preparedness and global health research.

Company
- About Us
- History
- Administration
- Board of Directors
Care to Cure
- Providing NHPs
- Scientific Research
Caring for Life
- One Welfare
- Bioculture Cares